Certifications & Approvals
Painfree8800 is a trusted medical device with global certifications and Korean FDA approvals
Global Certifications
CE
European CE Mark
EU Medical Device Conformity
2008
FDA
US FDA Clearance
FDA 510(k) Clearance
2009
KFDA
Korea FDA Approval
Korean Ministry of Food and Drug Safety approval
2020
NMT
New Medical Technology
Korean Ministry of Health and Welfare recognition
2013
Certification Details
CE
European Conformity (CE Mark)
EU Medical Device Directive (MDD) compliance certification
FDA
FDA 510(k) Clearance
US Food and Drug Administration premarket clearance
식약처
MFDS Medical Device Manufacturing License
Class 3 medical device manufacturing and sales approval
NMT
New Medical Technology Assessment Recognition
Scrambler therapy safety and efficacy recognition (MOHW Notice No. 2013-97)
Medical Device Classification
| Treatment Modality | Product Code | Class |
|---|---|---|
| Medical Laser Irradiator | A37020.01 | Class 3 |
| Transcutaneous Pain Relief Electrical Stimulator | A16010.03 | Class 2 |
| Ultrasound Stimulator | A16090.01 | Class 2 |
| Medical Electromagnetic Generator | A85020.01 | Class 2 |
| Low-frequency Stimulator | A16010.01 | Class 2 |
| Medical Thermal Device | A16150.01 | Class 2 |
Licenses & Patents
Medical Device Manufacturing Licenses

Medical Device Manufacturing License
- License Number
- 제 6094 호
- Issue Date
- 2018.05.23
- Issuing Authority
- Seoul Regional MFDS
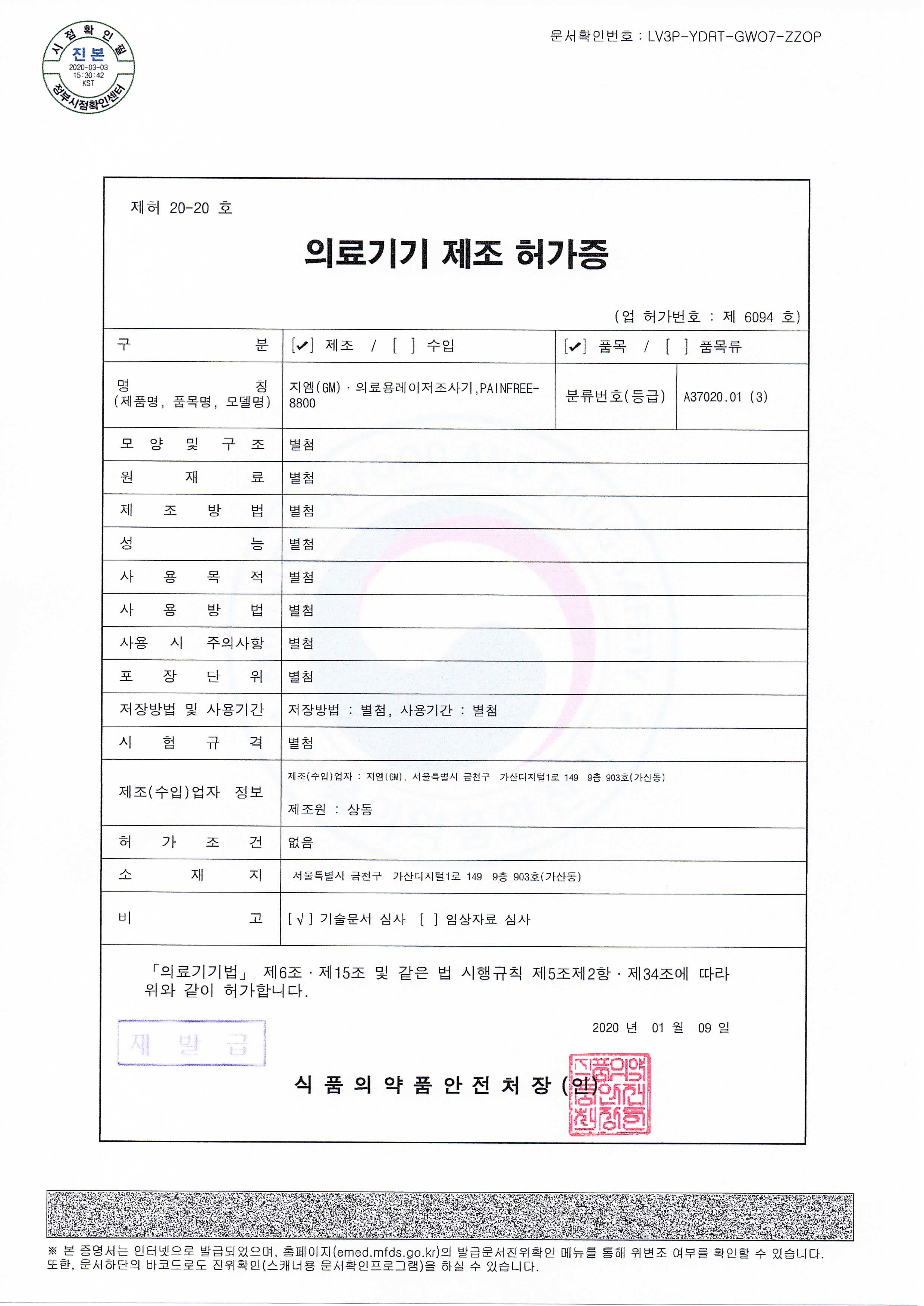
Medical Device Manufacturing Approval (PAINFREE-8800)
- License Number
- 제허 20-20 호
- Issue Date
- 2020.01.09
- Issuing Authority
- Ministry of Food and Drug Safety
Design Registration

Design Registration Certificate (Medical Electrical Stimulator)
- Registration Number
- 30-1048945
- Registration Date
- 2020.03.02
- Issuing Authority
- Korean Intellectual Property Office
Quantum Aqua Certification
KC
Quantum Aqua KC Certification
- License Number
- R-R-aq7-퀀텀Aqa
- Issuing Authority
- National Radio Research Agency (KC)
Certification Inquiries
Please contact us if you need certificate copies or additional information.
Contact Us